5.1 Biodiversity
Biodiversity refers to the number of different taxonomic or functional units of organisms living in a kelp forest. It is reported in number of species, number of functional species, presence, or absence of notable species.
Common indices include:
Species richness: A rarefied )or non-rarefied number of species found within a defined area (m2, ha, km2).
Diversity: A measure of the relative abundance and number of different species in an ecosystem - The Shannen Diversity Index and Simpson's Index are some of the most common.
Functional diversity): A variety of measures (number, evenness, dispersion) of functional units of species (e.g., body length, trophic group) in an ecosystem.
Endangered or iconic species: The presence or absence of species of particular interest, typically of economic, cultural interest or species that are listed on assessment schemes.
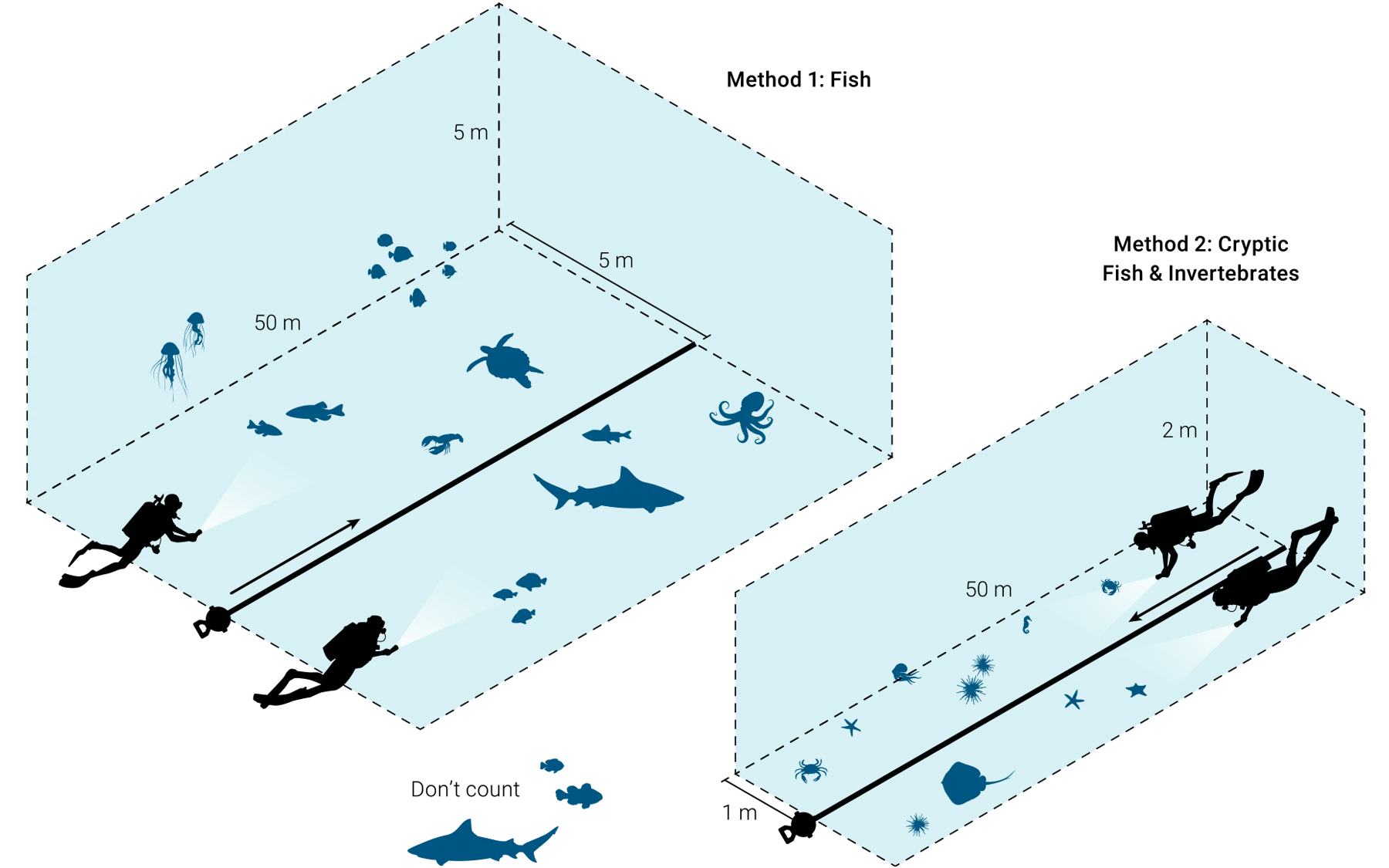
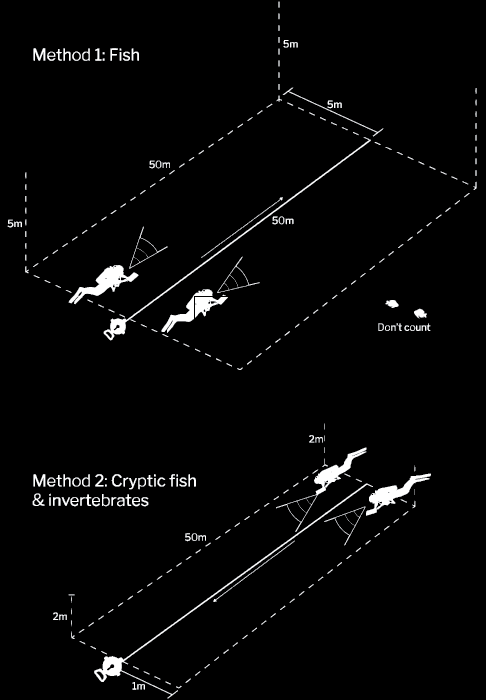
Figure 6. Reef Life Survey illustration of the transect method and dimensions used to count fish and invertebrates in water. This figure was adapted from Reef Life Survey (2023).
5.1.1 Biodiversity Measurement Options
The options for measuring biodiversity include:
- In-water visual surveys
- In-water video surveys
- Towed video surveys
- Automated video surveys
- Quadrats
- Epifauna
- eDNA
- Baited remote underwater video (BRUV) surveys
5.1.1.1 Video or Visual Surveys
We do not detail instructions for identifying organisms down to the species level, but assume that users are knowledgeable about their local taxonomy. If users are not familiar with the biodiversity present in their region, we suggest they consult the appropriate field guide or take photos and/or videos that can allow for retrospective identification.
Fish and mobile invertebrates, such as lobsters, may be identified using underwater visual surveys. These surveys may be done entirely visually, or they may also include a video recording to identify species partially or wholly. In-water surveys are much more time efficient as the values are complete at the end of the dive, but they may not be as accurate as video surveys that allow users to cross-reference a species’ identity.
Surveys are usually broken down into different sections of the water column. Benthic surveys focus on species living in the kelp, on or very near to the seafloor. Mid-water surveys capture species living above the kelp canopy. Open-water surveys capture species living near the surface; however, these surveys are not commonly used for kelp.
Users should record a species identity and abundance on a waterproof slate or datasheet. It may be helpful to prepopulate sheets with common species in the area to avoid writing down names while underwater.
We also suggest that users run a separate survey for benthic and cryptic species, ideally following the mid-water fish survey. These surveys should be run across the same transect as the in-water fish surveys. Instead of swimming in a straight line over the transect, users should take time to search crevices, nooks, overhangs, and other features that might contain organisms. It is highly recommended that users have an underwater torch for this component, especially if visibility is low.
Video surveys should follow the methods described in Section 5.1.2.2.
eDNA
5.1.1.2 DNA
Environmental DNA (eDNA) is a technique that detects the presence of a plant or animal DNA in a medium (e.g., water or soil). It can reduce sampling times and help identify species that were not visually observed during the survey. This fact makes it particularly appealing for monitoring rare, cryptic, highly mobile, or nocturnal species. However, eDNA has several core limitations:
- It can only detect presence and does not quantify abundance.
- Seawater is a well-mixed medium and the DNA therefore isn’t necessarily from a given location (e.g., a restoration site).
- It requires a DNA database for analysis, which might not be available for less common species.
- It is a technically complex and expensive process; for more details, see Gold et al. (2021) and Port et al. (2016).
5.1.2 Basic Instructions for Biodiversity Measurement
5.1.2.1 Visual transect surveys for fish and benthic diversity
Two divers should work together to efficiently sample fish, mobile invertebrate biodiversity, and benthic cover. These methods are based on the Reef Life Survey approach and will allow data to be compared to the Reef Life Survey dataset (Figure 6). A minimum of 5 m of underwater visibility is required to use this method effectively.
See Section 3.2 for choosing your starting location.
Projects should aim for two complete pairs of pelagic and benthic transects per dive.
Divers 1 and 2 swim while laying out the transect line for 50 m (see Section 3.2.1). Divers record pelagic biodiversity as they swim.
a. Diver 1 counts fish on one side of the transect line and diver 2 counts on the other side.
b. Divers only count fish within 5 m, horizontally, of the transect line and 5 m, vertically, from the sea floor. Divers should note if visibility is less than 5 m.
c. Record the size-category of total fish length (from snout to tip of tail, or longest distance, including for stingrays). The size-classes of total fish length commonly used are 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 20.0, 25.0, 30.0, 35.0, 40.0, 50.0, 62 cm, and above. The length of any fish longer than 62 cm should be estimated to the nearest 12 cm and individually recorded. Divers should practice identifying the length of objects underwater before completing these surveys.
d. Data is recorded directly on the data sheets.
e. If a species is unidentifiable, take a photo for later identification. Rare species may also be recorded.
Once complete, the divers swim the transect one more time and record invertebrates greater than 2.5 cm and cryptic fish, rolling the transect tape up as they go.
a. Divers should use the same formation as before but are now only counting individuals within one horizontal meter of the line and two vertical metres of the seafloor.
b. Divers should look under rocks and overhangs and into crevices and tunnels. We highly recommend an underwater torch.
c. Record the size-category of any organisms observed. Record carapace or test size for crabs, lobsters, sea urchins, or abalone. The measurement groups used are 2.5, 5.0, 7.5, 10.0, 12, 15.0, 20.0, 25.0, 30.0, 35.0, 40.0, 50.0, and 62 cm, and above. Estimate lengths of animals larger than 62 cm to the nearest 12 cm and record individually.
d. Do not record fish that you encountered during the previous survey. This survey only intends to capture fish that hide among rocks and the seafloor.
Divers may also record kelp density on the benthic transect. They can either count kelp holdfasts in the field or take a photo quadrat. We recommend taking a measurement every 5 m. Physical quadrats will provide more precise measurements; otherwise, a standard point and shoot camera positioned 50 cm directly above the reef captures an area of approximately 30 cm2. Once that transect is complete, repeat the survey on a newly placed transect, as described in Section 3.2 and Section 3.3.
5.1.2.2 Video surveys for fish and benthic diversity
If users are not confident that they can count and measure fish efficiently underwater, they may use video surveys instead. These surveys cannot replace the benthic biodiversity surveys.
The steps and formation are the same as for visual transects (Section 5.1.2.1), except:
- Locate a stereo video camera system (i.e., two video cameras mounted on the same unit and pointed in the same direction, as shown in Figure 7). See Goetze et al. (2019) for more details on sourcing the necessary components.
- Swim the same transect patterns, but use video cameras, as opposed to counting fish in real time.
- Turn the camera on before entering the water.
- Have someone clap while recording to allow the camera footage to be synced up (if using length measurement software).
- Show your fingers to the camera before starting each transect; show the number of fingers that corresponds to the transect number (i.e., one finger for first transect, two fingers for the second transect, etc.).
- Swim slowly along the transect line at a speed of three seconds per meter (20 m per minute).
- Once you have reached the end of the transect line, wave in front of the camera or point it at the surface to indicate the transect is over.
- Repeat this process for all the transects.
The videos may then be loaded into a software called EventMeasure which will allow you to identify and measure the length of each fish observed in the video. Only count fish in that 10 m x 5 m box used in the visual surveys. As discussed, users still need to complete the benthic and photo quadrat surveys separately. We advise to complete the video survey first to avoid startling any fish.
5.1.2.3 Quadrats and Biodiversity Surveys
Smaller animals or other organisms (i.e., epifauna) which grow or live on the kelp itself may require specific surveys because they are often overlooked in swim over surveys.
Larger epifauna (e.g., snails, sea stars, limpets, etc.) may be sampled using quadrats and can be counted using the same quadrats as those used to measure density.
Sampling of smaller epifauna (e.g., amphipods, isopods, and larval stages) usually requires removing the kelp plant, transporting it to the lab, and rinsing it over a sieve to collect the organisms, which can then be identified and counted immediately or preserved (e.g., in Industrial Methylated Spirit or similar) and processed at a later date. This sampling is destructive and kills both the kelp and the organisms. Therefore, it should only be done when absolutely necessary, and with as few kelps removed as possible.
5.1.2.4 Baited and Unbaited Remote Videos
Remote videos may be done without divers, which reduces their costs. Cameras are attached to an apparatus that contains an attractant to lure animals towards the camera, which is stationary. Because it is stationary, this method cannot be used to get an estimate of individuals per unit area, and data collected through this approach is only comparable to other data collected with the same method. Data collected using this method, rather being expressed per unit area, is often measured in soak time (i.e., how long the camera was in the water) or Max N (i.e., maximum number of species/individuals seen in a single video frame).
Users may select to use baited video cameras or unbaited video cameras. Baited cameras contain a food source (often oily fish or squid) that attracts predatory organisms towards the camera. Since this approach aggregates these animals, it biases the sample, and species counts from baited videos should only be compared to other counts obtained using baited methods. Unbaited videos result in a more random biodiversity sample, but should also only be compared to other counts obtained using unbaited approaches. See Langlois et al. (2020) for further details.
The baited remote underwater vehicle (BRUV) methodology described below focuses on animals near the bottom, but BRUVs may be modified to capture pelagic species as well, though that method is not described here.
Steps to Capture Remote Videos using a Baited Remote Underwater Vehicle (BRUV):
Preparation
a. Choose a suitable underwater camera and housing for your study. Using two cameras allows for length measurements to be gathered from the video footage.
b. Set up appropriate lighting, if needed, to ensure adequate illumination of the field of view.
c. (Optional) Determine the bait type and quantity to attract the target species.
d. Assemble the BRUV rig with the camera, bait, and any additional components such as weights, floats, or anchors.
Deployment
a. Select the study sites and transects based on your research objectives.
b. Record environmental variables such as temperature, salinity, and depth at each deployment site.
c. Deploy the BRUVs by lowering it to the desired depth, ensuring it is stable and well-positioned on the seafloor.
d. Allow the BRUVs to record for a predetermined duration, typically ranging from 30 minutes to several hours.
Retrieval
a. Retrieve the BRUVs using the attached line or marker buoys.
b. Carefully clean and inspect the equipment to ensure it remains in good working condition.
Video analysis
a. Review and catalogue the video footage, noting the start and end times, location, and any relevant observations.
b. Identify and count the target species present in a video frame considering factors like species behaviour, size, and abundance.
c. Measure environmental variables such as habitat type, complexity, or substrate composition, if relevant to your study.
5.1.2.5 DNA
See Gold et al. (2022) for a detailed breakdown of the process, but in short:
Ensure you have the correct primers for your target taxa, a comprehensive reference database, and the correct sampling and lab protocols to avoid spoiling your samples.
Run local pilot studies to determine how well the process works in your environmental context.
Run field surveys to visually assess species richness and validate the eDNA numbers in the project area.
5.1.3 Projected Costs and Comparison of Methods
Each method of biodiversity measurement has various associated costs (Table 13), as well as pros and cons for implementation (Table 14).
Table 13. Projected costs for biodiversity measurement options.
| Method | Cost |
|---|---|
| SCUBA Video Survey | Medium |
| SCUBA Visual Survey | Low |
| Towed Video | Medium |
| Quadrats | Medium |
| Epifauna | Medium |
| eDNA | Low |
| BRUVs | Medium |
Table 14. Pros and cons for biodiversity measurement options.
| Measurement Technique | Pros | Cons | Reference |
|---|---|---|---|
| In-Water Visual Surveys | Data available instantly Lower cost Better for benthic organisms | Less accurate Requires some training Must still carry a camera to identify unknown species Covers a smaller area compared to towed/automated | Edgar & Stuart-Smith, 2014 |
| In-Water Video Surveys | Higher accuracy Ability to check species’ identification | High processing time Some equipment and software required Cannot survey cryptic or benthic species Covers a smaller area | Smith et al., 2021 |
| Towed Video | Covers a larger area | Requires a boat Misses benthic and cryptic species Difficult in shallow depths | Galaiduk et al., 2017 |
| Automated Video Surveys | Covers a larger area May be automated | Expensive Range may be limited currently | Mallet and Pelletier, 2014 |
| Quadrats | Counts small and very small animals (e.g., epifauna) | Time-intensive Counting epifauna usually requires destructive sampling | Leclerc et al., 2016 |
| Epifauna | Counts very small animals (about 1mm) | Very time-intensive Destructive for both kelp and animals | Tuya et al., 2014 |
| eDNA | High accuracy for presence-absence Detects even if not visible, good for rare, mobile, or nocturnal species | High startup and processing costs Presence-absence only Correct reference libraries are needed Cannot associate results to specific square metre areas | Gold et al., 2021 |
| BRUVs | May attract rare species. No diver required May attract more benthic species | Cannot measure per unit area Unadvisable to compare to other sampling approaches | Cappo et al., 2006; Langlois et al., 2020 |