Figure 5.1 Flow chart of restoration methods
5.1 Restoration methodologies available
Project methodologies are not mutually exclusive, and projects may desire or need to combine approaches simultaneously or sequentially. Examples and considerations are given for the four main approaches to kelp forest restoration (grazer management 5.2, seeding 5.4, transplanting 5.5, and artificial reefs 5.6). All projects need to consider if the environment will allow for the growth of introduced kelp materials; these conditions may have shifted from previous baselines, and you will need to consider which kelp species you are working to restore (chapter 7). A common threat to kelp forest projects is overgrazing by herbivores such as sea urchins and herbivorous fish. Any project with overabundant herbivores will need to consider grazer management as an essential element of their project (chapter 5.2). If there are parent populations of kelp nearby, grazer management may be enough to restore a kelp population. Otherwise, projects will need to introduce reproductive kelp material into the ocean; you can achieve this either via seeding (chapter 5.4) or transplanting (chapter 5.5). Projects that are looking to enhance kelp forests as opposed to restoring them on natural reef may consider using artificial reefs, which provide new habitat for nearby kelp or act as installation sites for transplant and seeding efforts (chapter 5.6). We detail the advantages and disadvantages of these methods along with their key considerations in this chapter.
5.2 Herbivore management
Herbivores, i.e., grazers, often have strong interactions in shaping kelp forest ecosystems. Species such as sea urchins, herbivorous fish, and gastropods (marine snails) have historically been integral parts of kelp ecosystems and have consumed or removed kelp material at rates enabling it to regrow. Herbivores only become problematic to a kelp forest when their populations, and thus their grazing rate, outpaces the recovery of the local kelps. Herbivore population explosions on temperate reefs can occur because of multiple factors such as the loss of the herbivore’s predators, or strong recruitment events and/ or the expansion of the herbivore into a new habitat 1 2. Additionally, behavioral shifts from ‘normal’ grazing to ‘destructive’ grazing can occur due to reductions in local drift-kelp supply (Kriegisch et al., 2019). As herbivores are often natural components of the ecosystem, a value decision is made when choosing to control them or not. Whether this decision is justified will ultimately depend on their impact on kelp forests, the local community, and governance of the desired ecosystem state (chapter 3).
Herbivores can be managed by increasing mortality rates via culling, harvesting, rebuilding natural predators, or restricting them from a kelp forest. Here, we outline how to measure the state of grazing in your ecosystem and the options available to manage grazer populations. Due to the prevalence of urchins as the primary destructive grazer of kelp, the focus of this section will be on sea urchins, but we will also consider how you can extend these techniques to herbivorous fishes, an increasingly common problem. Gastropods are not covered as there are few examples of them preventing kelp restoration, although they do influence natural kelp recruitment 3 4.
For successful kelp restoration, grazing pressure needs to be reduced so that kelp growth and recruitment can exceed losses of kelp biomass. Kelp biomass production can be increased by either removing the grazer from the system or limiting its access to the kelp forest. Because herbivores, notably urchins, form alternate stable states in kelp forests (i.e., an urchin barren versus a kelp forest, chapter 2.1.4, box 2.3), the target density required to restore a kelp forest must be explicitly considered. This density depends on the local conditions, but large reviews have found that < 70 grams of urchin/m2 or < 2 urchins/m2 are general targets and can facilitate the return of a kelp forest 5 While having zero urchins is an unrealistic and often undesirable goal, initially reducing and maintaining urchin density as low as possible will provide the best chance for converting urchin barrens to kelp forests. A study in Western Australia found that a system transformed back into a kelp forest when herbivorous fish biomass was < 700 grams/m2 6, although this value needs verification in other locations.
Herbivore densities required for restoration are likely dependent on the ‘health’ of the local kelp forest, and positive factors such as high abundances of natural predators, low sedimentation loads, low nutrient pollution, nearby kelp adults, and low harvest rates can all help to create more resilient kelp beds, which may tolerate higher numbers of herbivores 7 8). If grazing is a consistent problem, it may be best paired with transplanting as opposed to seeding, because adults can withstand greater grazing pressures than juveniles.
5.2.1 Herbivore harvest, culling, or translocation
Herbivores can be removed from an ecosystem by harvesting, culling, or translocating them. The logistics of harvesting and culling are often the same (e.g., hand removal), but culling processes do not have an end use (e.g., food or fertilizer). In situ culling involves crushing or puncturing the test (i.e., shell of the sea urchin) and leaving it in the water. If the urchins are being used as a food resource, they are first removed from the water, then processed on the boat or on land. If you are transporting the urchins, the animals are transported unharmed and released into an alternate location. You can conduct fish culling via nets—usually seine nets—or possibly by hooks or spearfishing.
5.2.1.1 Mechanical culling of urchins
There are several different tools for removing sea urchins in situ, including hammers, rollers, crow bars, hooks, tridents, or poles. You can use these tools while SCUBA diving, freediving, or in some rare cases from the surface. You kill urchins when you break their test (i.e., shell), alternatively, a hole, > 2cm x 2cm effectively incapacitates them. Selecting which combination of in-water access and tool for culling is mostly dependent on the skillset of the people doing the culling. The tool of choice will depend on diver preference, but we suggest rollers and rakes in flat areas and crowbars and hooked poles to reach into crevices.
Anyone who can safely work underwater can conduct urchin culling. This range of people includes professional divers (commercial fishing fleet), scientists, and qualified volunteers (Northern California: Bull kelp). Indeed, many projects have successfully recruited volunteers to cull urchins. As the user’s skill level increases, so does their culling rate, and it may therefore be most beneficial to focus on training the same people and having them work consistently as opposed to recruiting new people (i.e., volunteers) each time. However, recruiting and managing volunteers is also a laborious process that requires a dedicated coordinator. So, while the culling labor may be “free,” you still require other inputs. As working underwater is an inherently dangerous process, it is imperative that all people involved are properly trained and, if appropriate, that the project has insurance for people to work underwater. Industry divers (e.g., commercial abalone divers) are often motivated to remove sea urchins, as their fisheries benefit from kelp recovery (Haida Gwaii: Gwaii Haanas & Tasmania: Urchin control).
Culling urchins in combination with dive harvesting of other species, e.g., abalone, is also practiced but typically only effective at small “incipient barrens” scales as you spread your focus across two tasks. Researchers in Japan and Norway are currently trialling a new approach using a vacuum pump. This approach allows a diver to stay underwater for longer, as they do not have to manually transport their catch to the surface. Divers may dive on SCUBA, hookah, or freediving, as diver experience permits. All removal strategies should be consistent in the locations targeted, with efforts focused on select locations over time, as opposed to efforts spread too thin (Southern California: Palos Verdes).
5.2.1.2 Chemical control
You can use a compound called quicklime (Calcium Carbonate, CaO) to cull sea urchins and other echinoderms (Norway: Quicklime). Quicklime is typically a by-product of lime production and is inexpensive to acquire (< 10$/ kg). When quick lime is put in contact with water, it creates a strong base that causes lesions in the tests of sea urchins (CaO + H2O -> Ca(OH)2. Quicklime is lethal to echinoderms and can affect abalone 9 but has limited effects on other organisms, especially mobile ones such as fish 10 11 12. You apply quicklime from the surface or underwater while diving, and the particles (0–2 mm in size) distribute into the water column. These pieces then float to the benthos and dissolve the urchin test upon contact. Not all particles reach the seafloor, but all particles eventually dissolve, and the base is diluted in the seawater in less than an hour.
Using quicklime is attractive and low cost, can be applied without divers, and can cover a large spatial area. However, it is controversial, as it involves some level of collateral damage. Collateral damage can be minimized by targeting areas that are mostly populated by urchins or by using divers to deploy quicklime closer to the seafloor. There have been some reports that quickliming is less effective in colder waters, but new research shows that liming is not significantly impacted by temperature at a low range (2° vs 10°C, 13) but may be less effective at higher temperatures. Strong consideration must be given to determine if quickliming is justified and socially permissible in each situation.
5.2.1.3 Urchin trapping and baiting
Urchin trapping or baiting increases the densities of catches of targeted urchin species and allows for more efficient urchin removal. In Norway, trapping was found to be an economically viable alternative to harvesting urchins in comparison to diving 14. This Norwegian study evaluated bait composed of fish and algae and found that fish bait attracted more diversity and by-catch compared to algae. The most successful trap was a round collapsible trap, and they suggested that traps sit in the water for three to eight days. You can also use traps and bait to aggregate urchins in one location and facilitate quicker culling efforts.
5.2.1.4 Removal of fishes
While there has been little focus on managing herbivorous fish populations, the topic is expected to become more relevant as expansion of herbivorous tropical fish into temperate waters is facilitated by warmer waters 15. In shallow waters (< 5 meters) in Japan 16, herbivorous fish removal has been accomplished using seine nets. However, this technique does not discriminate by species and requires a high level of skill to conduct. While you can return bycatch species to the ocean, stress and mortalities are inevitable. These factors can be mitigated by proper animal care procedures but cannot be eliminated entirely. As a result, a great deal of care and justification should be required before attempting to seine net an area for culling purposes. Spearfishing is a low by-catch alternative for removing herbivorous fish but requires more skill and removes fish at a much slower rate than seining.
| Box 5.1 Harvesting herbivores for food |
|---|
| As both sea urchins and fishes are consumed as human food, there is the potential to remove these species from target sites and sell them to cover project costs, or even to turn a profit. The market is most developed for sea urchin roe, or “uni,” which is a common food item in Japan, the Mediterranean, and increasingly worldwide. There is no known international market for the common herbivorous fishes, but some are consumed locally, and perhaps new marketing campaigns could encourage their consumption. Additionally, urchin barrens may not contain sufficient food sources to sustain healthy urchins, and the quality of urchin roe suffers and is not worth selling. An innovative market driven solution created to address this problem is “urchin ranching,” where people collect the poor-quality urchins of a particular size class from the ocean, culture, enhance, and then sell them. This process adds additional costs but can create higher value and market price for “uni” and may prove to be an economically viable venture. If such operations prove unprofitable, they could be run as philanthropic projects, or government subsidies can help fund project costs (Tasmania: Urchin control). |
5.2.2 Localized urchin exclusion
While less commonly used, in some cases it may be necessary to exclude urchins from kelp habitats. It is possible to create herbivore exclusion structures using f loating nets, rigid fences, bubble cages, or with other habitat formers such as octocorals 17 18. One project also succeeded by planting artificial kelp alongside live transplants. The artificial kelp creates a whiplash effect in the current and can deter urchin grazing 19. This technique might be most useful for targeted small areas in order to protect outplants or allow for new recruits to grow.
5.2.3 Positive species interactions
Projects will never achieve lasting results without an effective and long-term solution to overgrazing. The methods described above can require regular intervention and are thus logistically challenging as well as costly. Ideally, an ecosystem would be able to sustain itself without regular human intervention. The best way to achieve this goal is by ensuring that the (non-human) predators of herbivores (e.g., fishes, lobsters, sea stars, otters) are present in sufficient numbers. Because most predators have declined due to human harvest, marine protected areas (MPA) or reserves that limit or eliminate harvest pressures can help achieve this goal and ensure healthy predator populations. Synthesized evidence suggests that MPAs can indeed restore or maintain kelp forests, but there remain instances where increases in predators’ numbers do not result in increases in kelp populations. Therefore, MPAs cannot be considered a universal fix for kelp restoration but can certainly play an important role in maintaining, if not restoring, healthy kelp forests 20. Once predators are re-established, limited take and sustainable fisheries policy can help ensure their populations do not fall again.
5.2.5 Key considerations for herbivore control
Urchin types: Not all urchins are equal in creating barrens; some species are more prone to creating barrens than others. It is not advisable to indiscriminately remove sea urchins but rather to consider the dynamics in your system and choose your management strategy accordingly.
For example, scraper species (e.g., Centrostephanus rodgersii) are more likely than opportunistic grazers (e.g., Heliocidaris erythrogramma) to create urchin barrens, as long as there is a sufficient supply of drift algae. Alternatively, more mobile species such as Strongylocentrotus droebachiensis may be likelier to cause barrens. In addition, some species are important to fisheries and subject to management regulations (e.g., Mesocentrotus franciscanus).
Consistent intervention: Herbivores can grow at fast rates, disperse as larvae over hundreds of kilometres and over tens of metres as adults, and only require relatively low densities to maintain a barren (as low as ~70 grams of urchin biomass m-2). As a result, it is essential that work to remove/manage herbivores is consistent over space and time. If you distribute management efforts across too many locations or only once, it is often not possible to reduce the herbivores to the critical threshold required to restore a kelp forest.
We recommend that projects work in concentrated locations and plan (including budget) to maintain a site for multiple years before commencing.
Proximity to existing kelp sources: Because kelp species are typically short dispersers, we recommend that projects select culling sites that are close to existing kelp forests. This proximity will help facilitate natural repopulation of the kelp forest.
Removal strategies: You should first target herbivore removal efforts at the edges of the barren and then work towards the centre as urchin numbers are reduced: i.e., propagate kelp recovery from adjacent beds to maximise local sources of kelp propagules to recolonise barrens once urchin densities have been sufficiently reduced.
Prevention and pre-collapse: Because it is easier to prevent an urchin barren from forming than reversing an existing barren, it is more cost efficient to be proactive and to manage herbivores in a degrading but not collapsed kelp forest (chapter 2.1.6).
Site topography and habitat: Sites with natural barriers such as sand stretches and rock formations can help prevent urchins from encroaching on a site. Therefore, selecting these sites to cull urchins may increase the chances of success.
Ease of culling: It will be easier to cull urchins from sites that are flat, where you can sweep the urchins off the rock or ensure they are impacted by quicklime. Otherwise, it is difficult to locate and remove all urchins from sites that contain hiding places for urchins such as boulders or other crevices, in which case multiple culling efforts are often required to remove individuals missed on the first pass. Baiting urchins may draw them out into the open and congregate them to facilitate easier culling.
5.2.6 Further reading
21 Ling, S. D., Kriegisch, N., Woolley, B., & Reeves, S. E. (2019). Density—dependent feedbacks, hysteresis, and demography of overgrazing sea urchins. Ecology, 100(2), e02577.
22 Ling, S. D., Scheibling, R. E., Rassweiler, A., Johnson, C. R., Shears, N., Connell, S. D., ... & Johnson, L. E. (2015). Global regime shift dynamics of catastrophic sea urchin overgrazing. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1659), 20130269.
23 Tegner, M. J., & Dayton, P. K. (2000). Ecosystem effects of fishing in kelp forest communities. ICES Journal of Marine Science, 57(3), 579-589.
24 Keane, J., 2021. Resetting urchin barrens: liming as a rapid widespread urchin removal tool.
5.3 Sourcing kelp material for seeding and transplantation
Sourcing kelp material is a key step in seeding (chapter 5.4) or transplanting (chapter 5.5) kelp for restoration. You can source kelp material for kelp restoration projects in two ways: 1) direct use of wild materials (e.g., transplants, spore solution, and spore bags; see Sydney: Operation Crayweed) and 2) lab culturing (Korea: Seaforestation project, Appendix 1). Here, we classify culture methodologies as those that increase the amount of reproductive material (i.e., more individuals regardless of life stage). Unless you use a commercial seed stock, all these processes start from a wild population. Each of these methods has several pros and cons (Table 5.2) that we describe in more detail later in the chapter.
5.3.1 Genetic diversity in restoration
It is important to consider the genetic diversity of the kelp material used in all stages of the restoration process. Higher genetic diversity in a population can increase the likelihood of surviving a stress event 25 and allows populations to adapt to changing environmental conditions. Incorporating genetic diversity into restoration will also preserve different phenotypes 26 and unique evolutionary lineages 27 and can also influence associated biodiversity and biomass, as well as ecosystem functions 28. While not all projects may have access to sequencing infrastructure, genetic diversity can be considered in the process of selecting the parental kelp.
When collecting seed material from donor populations, one can ensure that a sufficiently large number of different individuals are sampled to obtain a more genetically diverse seed stock (a general rule is 20–50 individuals). Collecting from different spatial areas of the population can also help ensure higher genetic diversity as individuals in close proximity (< 10 km) are more likely to be genetically similar 29. It is also important to consider biosecurity (chapter 4.2.2) and the match between the environmental conditions of the donor and outplanting sites when selecting kelp for restoration.
In most cases, restoration aims to re-establish populations that are most similar to those lost in the past. However, more forward-looking restoration strategies to increase resilience to climate change are increasingly explored in chapter 7 30.
Table 5.2 Pros and cons of wild versus cultured materials
| Pros | Cons | |
|---|---|---|
| Wild materials | More affordable if commercial culture and/or facilities are not available Quicker immediate timeline if facilities are not available Less equipment required Lower chance of biosecurity issues | Higher pressure on wild population Collections must occur close to time of restoration Less opportunity for genetic selection |
| Cultured materials | Less pressure on wild populations Can store cultured material for later (create a seed bank) Easier to work with genetic selection (chapter 7) Can produce large batches Can restore anytime not only when wild populations are reproductive | Requires more equipment, resources, and technical knowledge Higher potential biosecurity issues |
Figure 5.2 Flow chart for sourcing kelp material for restoration
5.3.2 Kelp collection
If you are not using a commercial stock, you must collect the kelp material to use for seeding or transplanting. Transplanting involves collecting the entire kelp, including the holdfast, while in seeding you only need to collect the reproductive tissue located on the blades (the sorus, Fig. 5.2), which does not kill the individual.
5.3.2.1 Collecting kelp for transplantation*
The kelp thallus (i.e., the entire individual) is primarily composed of three sections: the holdfast, the stipe, and the blades. Some species also possess a single or even multiple floats that allow the plant to stay upright in the water column. Sourcing material for transplantation involves the collection of the entire thallus. Holdfasts provide the only attachment point, and thus a critical aspect of the collection procedure is the detachment of the holdfast from the rocky substratum. Notably, kelp cannot be grown from cutting or fragments taken from other individuals.
Depending on the species and how strongly attached the holdfast is to the reef, holdfast detachment can be done via snorkel or SCUBA diving. The diver needs to introduce a knife or flat blade (abalone knives are ideal but large dive knives also work) between the bottom of the holdfast and the rock and detach the entire holdfast. It is important that you insert the knife along most of the diameter of the holdfast and reduce damage by ensuring most/all of the holdfast is detached in one piece.
You can bag detached kelp in the field using mesh-bags for ease of transportation. For most, if not all, species it is important that transplantation to the recipient site occurs within a few hours or at least the same day of collection. It is also important that you keep kelp shaded, cool, and moist during transportation to the recipient site. Keeping them in the mesh-bags used for collection and covering them with towels/tarpaulins works very well. If short transport time is not possible, kelps can be kept in mesh bags in the ocean overnight (e.g., tied off a dock) or stored in a large aquarium with aeration and water flow.
5.3.2.2 Collecting kelp material for seeding and culturing
We do not provide detailed instructions for culturing each type of kelp. Instead, we provide a set of general recommendations that work well for Laminarian species (also see gametophyte review 31). Any specific details below may not work for all species and are only described as starting points if species-specific information is not available. You may find a list of persons conducting kelp restoration of your target genera at kelpforestalliance.com.
Fundamentally, the culturing process typically requires releasing spores from reproductive tissue collected from the field, with spore release usually being triggered by ‘stressing’ the collected tissue through a process of exposure to low and then high light and/or mild drying before rehydrating. The released spores are then maintained under stable light and temperature conditions, and over the following days/weeks they are allowed to mature to subsequent life stages.
One critical and consistent aspect of kelp culturing is the importance of cleanliness and avoiding contamination. Common and problematic contaminants of kelp cultures include diatoms, flagellates, some molds and other fungi, algae, and bacterial growth. Reproduction in most kelps typically peaks during certain times of the year. You will need to research the appropriate time to collect kelp material depending on your target genus and region.
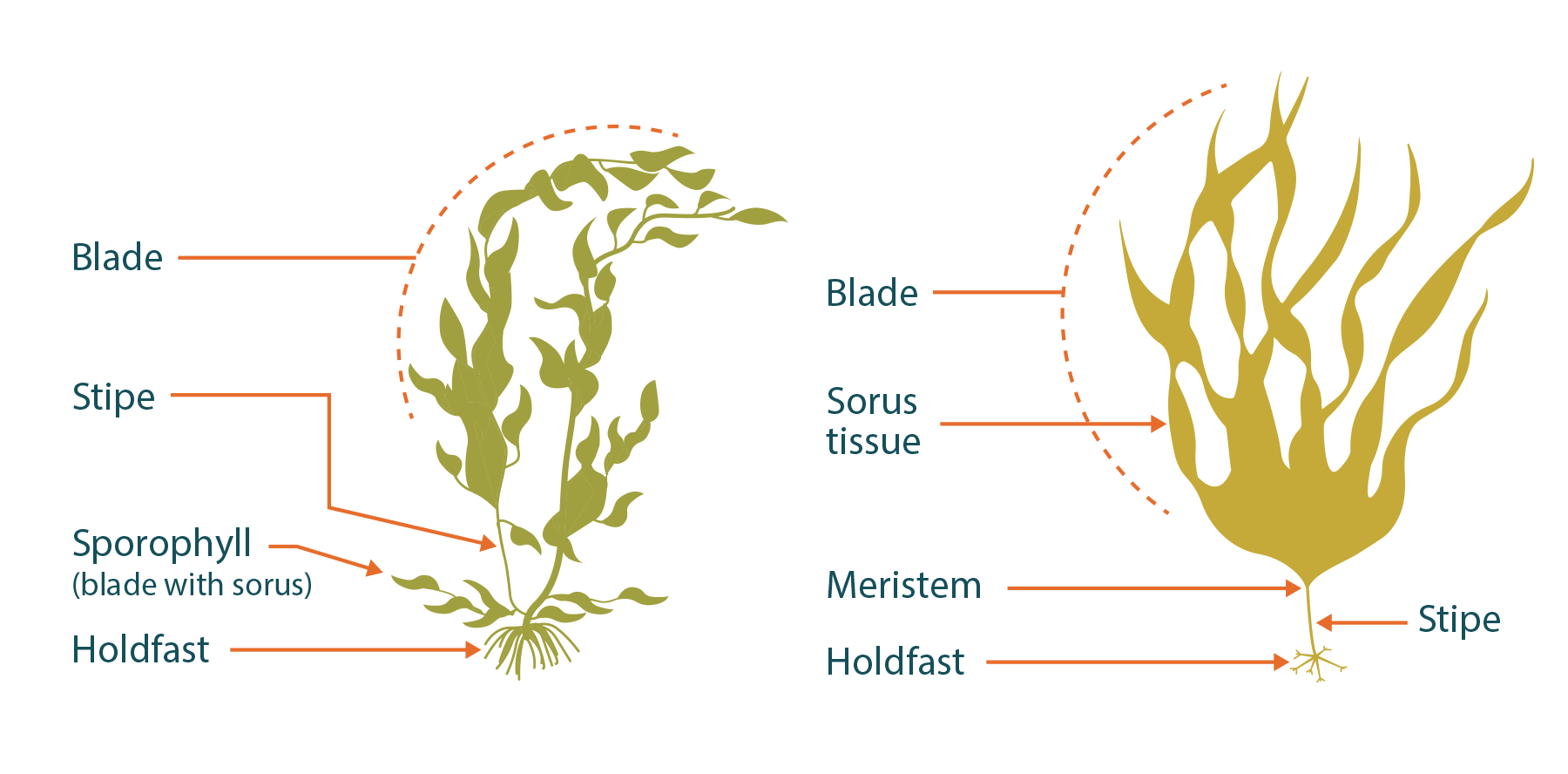
During collection, you are targeting the sorus tissue (sori for plural). Depending on the species, the sori are located on the blade or stipe, but we suggest targeting the blade tissue that is least destructive to the kelp (Fig 5.3). Within the sorus tissue is the sporangia, which contains the spores used for kelp reproduction. This tissue is typically held in the middle of the blade and is slightly raised and often dissimilarly coloured to the surrounding tissue (Fig. 5.4). While you can identify sorus tissue in the field, it is easiest to collect the entire blade and transport it back to the lab. When collecting blade tissue, you will want to look for healthy kelps.
Collecting blades
- Ensure you are collecting during the reproductive season
- Check local water conditions (e.g., tides, current, swell)
- Collection materials
- Choose your collection site
- Target healthy kelps
- Try to minimize damage to the kelp
- Take only what you need, do not over harvest
- 20–50 plants are suggested for creating a gametophyte solution
- Transport your collected material back to the lab
- Keep at water temperature from which the individual was collected
Figure 5.3 Anatomy of kelp; illustration by Jon Ferland
Figure 5.4 The sorus. This tissue is typically held in the middle of the blade and is slightly raised and often coloured a shade darker than surrounding tissue; © Ralph Pace
5.3.3 Spawning the sori and creating a gametophyte solution
We discuss the specific instructions to create a spore or gametophyte solution for a model species (Laminaria digitiata) in Appendix 1. The exact details of these steps (e.g., temperatures, nutrient concentrations) differ among species and regions; therefore we recommend consulting local experts (e.g., universities, culture facilities) when in doubt.
5.3.4 Applying spore/gametophyte solution to substrate or the environment
Once you have created your spore, gametophyte, or sporophyte solution, you will next want to apply it to your substrate of choice (rope, hard materials, directly into the ocean, see chapter 5.4 and 5.5). For rapid deployment, you will want to wait a minimum of 24 to 48 hours to ensure the spores or gametophytes have settled before introducing this material into the ocean (see considerations 5.3.6). During this time, it is important to maintain the environmental conditions (temperature, light, nutrients, salinity) suitable for growth.
Culturing to sporophytes (juveniles/adults)
You may choose to culture your stock further and grow it to a juvenile or adult sporophyte stage. Taking this step can be advantageous, as optimal environmental conditions and a lack of grazing generally increase survivorship. Survival is also greater for more mature kelps than for younger individuals. While advantageous, it also requires more time in culture and thus more resources. We do not cover the steps required to grow your gametophytes into adults as that is beyond the scope of this guide (i.e., aquaculture), but see Flavin et al. 2013 32 for a comprehensive description on culturing (including steps covered here).
5.3.5 Spore bags
If you are using spore bags, you may skip many of the steps outlined here. When using spore bags, take the section that contains the sori (blades or holdfast), dry them for ~12 hours in a shaded, cool, well-ventilated area, and then add them to the bag material directly. While transporting, minimize heat stress by using damp cloth and shading from the sun. Do not immerse in water as this will trigger spawning. Once you deposit the bags in the ocean, the ocean water should initiate the previously described spawning process. Be careful getting the blades wet beforehand, as you may prematurely induce spawning.
5.3.6 Key considerations for sourcing kelp material
Seasonal timing: It is best to mimic the natural reproductive cycle of kelps.
Processing: It is best to extract the reproductive material as early as possible after collection and from the cleanest part of the blade.
Culture time: If outplanting sporophytes, cultivate for enough time to allow developing sporophytes to grow past more vulnerable life stages (1-2 months, few cm length); NB: Kelps grown to sporophytes and left too long in the lab may become too adapted to optimal lab conditions and perform poorly after deployment.
Water flow: The cultivation setup should try to mimic water flow from the collections sites so that sporophytes develop sufficiently strong holdfasts.
Outplanting timing: You should outplant into the field during periods with high nutrient availability (winter/spring), adequate water temperature, and during times of low grazer abundance or algal competitors.
Target plants: Try to collect younger kelp, as they may be most fit for reproduction and there is evidence that collecting larger, older kelp can be more harmful to the natural population.
5.3.7 Further reading
33 Flavin, K., Flavin, N., Flahive, B., 2013. Kelp Farming Manual: A Guide to the Processes, Techniques, and Equipment for Farming Kelp in New England Waters.
34 Rolin, C., Inkster, R., Laing, J., Hedges, J., & McEvoy, L. (2016). Seaweed Cultivation Manual. Shetland Seaweed Growers Project 2014, 16.
35 Veenhof, R., Champion, C., Dworjanyn, S., Wernberg, T., Minne, A., Layton, C., Bolton, J., Reed, D., Coleman, M., 2021. Kelp gametophytes in changing oceans.
36 Merrill, J.E., Gillingham, D.M., 1991. Bull kelp cultivation handbook. [National Coastal Resources Research and Development Institute], [Portland, Or.].
37 Alsuwaiyan NA, Mohring MB, Cambridge M, Coleman MA, Kendrick GA, Wernberg T. 2019. Protocols for the experimental release of kelp (Laminariales) zoospores. Ecology and Evolution, 14: 8387-8398.
5.4 Seeding
Seeding is a common approach for ecosystem restoration in terrestrial systems but is currently less commonly applied in the marine environment. Broadly defined, seeding involves dispersing and/or growing the juvenile life stage (i.e., seeds, gametophytes, propagules, zoospores) of the kelp into the ocean. Seeding is advantageous because it is less resource intensive than transplanting, seeds can be grown in large quantities, selective breeding can choose desirable traits, and it has lower impacts on wild populations of kelps. Conversely, the microscopic life stage of kelp is more sensitive to disturbances like pollution, grazing, and waves than the macroscopic and adult life stages. When seeding, you must consider your seed source (chapter 5.3) as well as how and where you distribute them.
You can introduce the seed material into the environment in two main ways: direct release of propagules or ex situ seeding on substrata and outplanting. Propagules that are directly released into the environment are expected to settle on available natural rocky substrata and mature. You will disperse seed material that you have produced ex situ in the environment together with its vector substrata (i.e., rocks, ropes) and may produce new zoospores once reaching reproductive maturity.
If the substrate is covered by other organisms that would prevent kelp growth (e.g., coralline algae, turf), projects can work to clear the substrate prior to seeding. Clearing of the substrate can be effectively achieved by using a high-pressure air gun, scraper, or grinder. While air guns and grinders are more effective methods over large areas, they are also very costly and intensive.
5.4.1 Spore bags
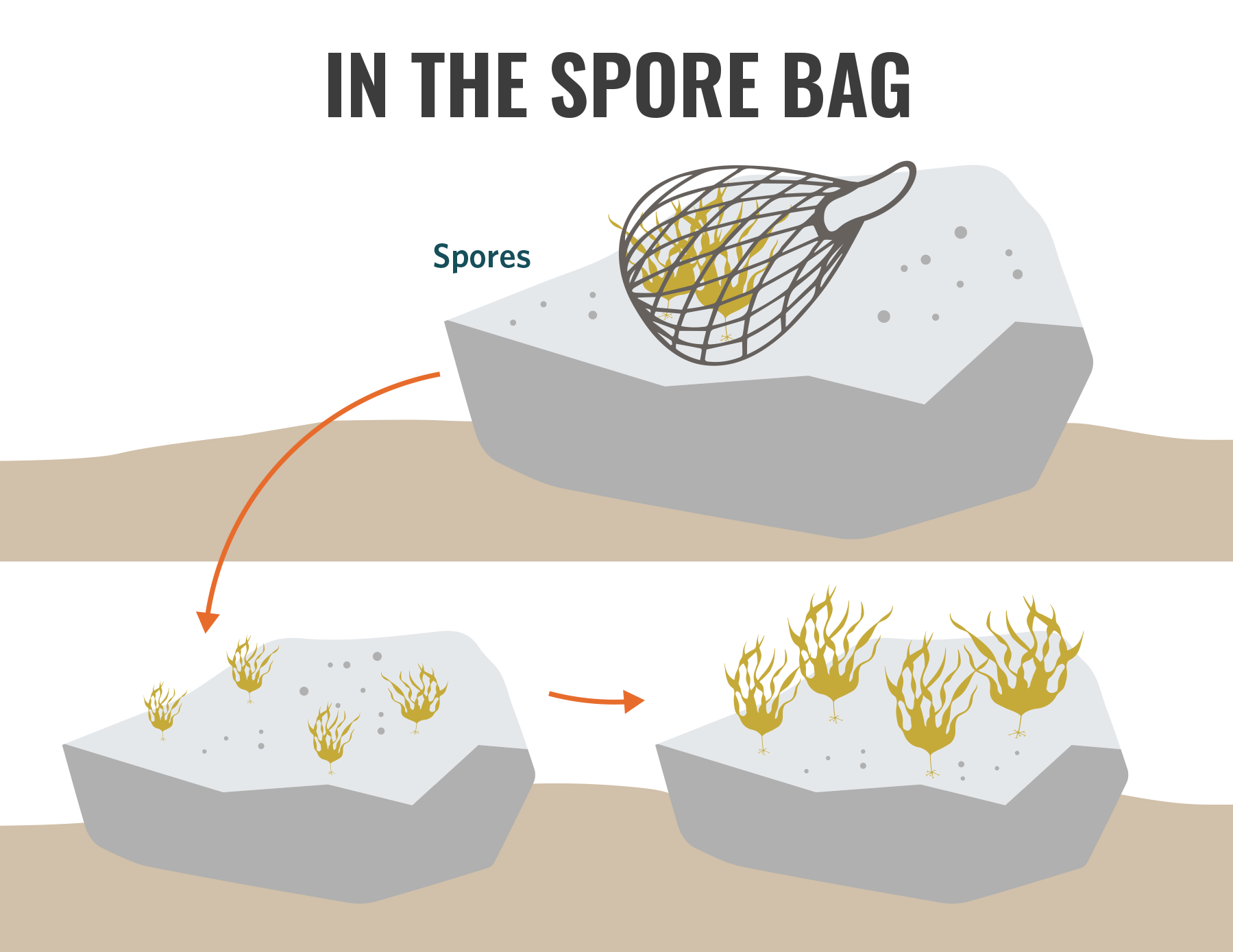
Spore bags (i.e., seed bombs) introduce reproductive material (kelp material with sori) into the environment to allow for the natural release of zoospores that can settle onto available rocky substrate. Spore bags consist of mesh bags filled with mature blades and are either placed on the substrate or suspended in the water column (Fig. 5.5). The parent material can either consist of wild collected, unprocessed kelp blades or of processed blades that you have prepared to induce spawning (chapter 5.3.5). Once you fill the bag, you attach it to a rope with one end anchored on the sea floor and the other on a float. The spores are then distributed across the benthos as the blades spawn. Following the spawning event, you should remove the bag, rope, anchor, and float from the ocean.
5.4.2 Seed lines
Seed lines are most used in aquaculture, but you can also apply them in a restoration context. Lines are typically made of nylon, but interest is growing in using biodegradable materials as well (e.g., cotton, though it is important to consider that some natural fibres require pre-treatment such was autoclaving or soaking in seawater). First, you inoculate seed lines with spores in a culture facility and then grow them out in the lab or in the field. Inoculation can involve spraying a spore solution on coils of lines that are out of seawater or adding the spore solution to seawater containing submersed lines. Grow out involves holding lines in tanks with clean filtered seawater. Once the lines are ready for installation, you will suspend them in the water column using a series of buoys and anchors. Small seed lines can be wrapped around larger ropes. A benefit of floating lines is that the kelps are free from urchin grazing and may have higher survival rates. These adults are then the propagule source for the future generation and can seed the benthos beneath the seed lines. Lines are typically removed after one spawning event as the line material degrades due to wear and tear.
Figure 5.5 Spore bags; illustration by Jon Ferland
Figure 5.6 Inoculating hard substrate; illustration by Jon Ferland
5.4.3 Attached to a hard substrate
You can apply the principles described above for inoculating hard substrates with kelp spores as a seeding vector. Rocks and stones are the most common substrates used for this purpose. A newly developed approach, termed “green gravel” (Norway: Green Gravel, greengravel.org) works by inoculating small stones (i.e., gravel or pebbles) with a spore culture, growing them to a young life stage in the lab and then dispersing them into the environment (Fig.5.6). You can disperse the stones by dropping them off the side of the boat, eliminating the need for scuba divers, while increasing scalability and reducing associated costs. You can apply a similar approach in situ by placing the settlement substrate underneath the canopy of reproductive kelp plants during the spawning period. The released spores naturally settle on the available substrate, and you can then collect and transport them to the restoration site.
The new kelp recruits will grow throughout the growing season and ultimately spawn within the new habitat. Depending on the size of the stone used, the kelp holdfast may also overgrow the vector material and attach directly to the sea floor.
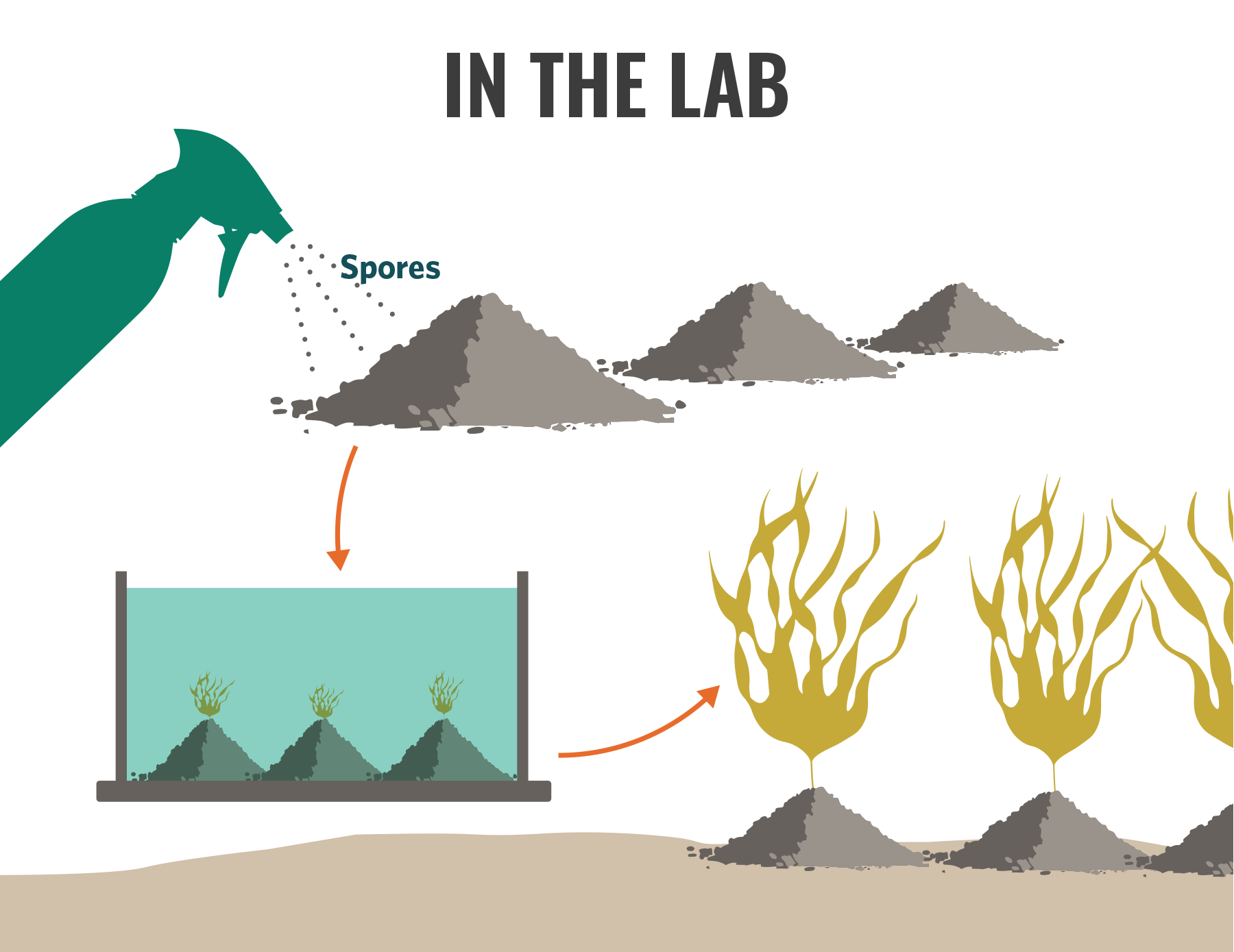
5.4.4 Direct dispersal
The direct seeding approach is the most different from the others and the one least tested. Direct seeding simply involves distributing a kelp culture directly into the water column or on the benthos in intertidal habitats. Subtidally, this work can either be done from the surface or underwater using a hose or other apparatus to “spray” the seafloor (Fig. 5.7). When working intertidally, the gametophytes/sporophytes are expected to attach to the rock before being washed away by the incoming tide.
Figure 5.7 Direct dispersal; illustration by Jon Ferland
Table 5.3 Pros and cons of seeding methods
| Pros | Cons | Reference | |
|---|---|---|---|
| Spore bags/Seed bombs | Limits grazing of source tissue Relatively cheap at small scale Invisible from surface | Generally requires wild harvest Requires removal of higher amounts of material Suited for low current areas Difficult to assess if propagules have settled | (FIRA, 2020)38 |
| Seed lines— floating | Established protocols from aquaculture Covers large area Kelps are protected from urchin grazing (but not fish) Applicable over large scales | Costly deployment Requires removal of material Suited for low current areas Needs to be deployed for longer time periods than aquaculture lines to reach reproductive stage | (Shaw et al., 2018)39 |
| Seeded substrate—cultured (e.g., green gravel) | No divers required No clean up required Lower cost | Suited for low wave/current areas May be some introduction of foreign material | (Fredriksen et al., 2020)40 |
| Seeded substrate— wild spawn | No culture required Using larger substrate pieces (e.g., rocks, boulders) can make it more suitable for high wave areas | Requires divers Stones/substrate may be expensive and hard to source in some areas | Japan: Hainan transplants |
| Direct dispersal | Low equipment required Very little material removal required | Need a culture Smaller area covered if performed by diver Special equipment required | (FIRA, 2020)41 |
5.4.5 Key considerations for seeding
Grazers: Sea urchins are problematic for many seeding methods; in such instances, projects may need to 1) manage the grazer population (chapter 5.2) or 2) select areas or seasons with low grazing pressure. Elevated reefs or areas isolated from urchin habitat by sand patches may provide areas with lower urchin grazing pressure.
Available surface/site selection: Propagules will need suitable surface to settle, adhere, and grow on. You can choose areas so that they have: 1) low sedimentation rates, 2) low cover of competing species (e.g., turf, bryozoans), 3) suitable rocky reef, 4) optimal wave exposure. In some cases, the addition of substrates can overcome this limitation.
Turf algal and sub-tropical macroalgal reefs often fluctuate seasonally in cover, so targeting seeding for periods when cover is low may help address the challenge of competition for settlement surface. Suspending culture lines of seaweeds in the water column can also shade out some of these competing algal species, or if you attach the lines on the seafloor, the larger kelps could scour off the turf.
Selecting substrates with a high surface rugosity can also help improve the strength of attachment and decrease the likelihood of detachment.
Seasonality: Most kelp species have optimal periods of reproductivity during the year. Therefore, you need to time seeding with this period or maintain efforts for multiple years to cover multiple reproductive seasons.
Wave exposure/currents: High wave exposure and/or currents will increase the difficulty of successfully seeding an area. You should try to ensure that work conducted in these environments releases the propagules close to the substrate. While difficult, working in these areas may indeed be beneficial as high wave action can deter sea urchins, and kelps grow faster in these areas. Storms may also be seasonal, and you should plan seeding attempts for calm weather seasons.
5.5 Transplanting
Transplantation (i.e., transplanting) of kelps has been the most common active method used in past kelp restoration efforts. We define transplanting as the introduction of the adult life stage of kelp into the marine environment, specifically on the benthos. The ultimate goal of transplanting is to provide a canopy that may facilitate kelp recruitment from nearby populations and/or allow propagules to settle and grow into adults. As such, the long-term focus is the survival of the second generation, not the initial transplants.
Transplanting is advantageous because it uses older life stages that are typically more resistant to stressors such as grazing, pollution, and waves, and thus it has higher survival rates than seeding, creates a canopy that facilitates recruitment and growth of juveniles, and is very targeted in its placement. Conversely, transplanting is resource intensive, may not be viable at large scales, and often requires kelps sourced from local populations. When choosing the best transplanting method, it is important to consider the local conditions and trial approaches before scaling up.
You may attach kelps to the rock in many ways, but all approaches work to secure the holdfast to the seafloor and, depending on the species and method, the kelps may overgrow the substrate and attach to the seafloor. See chapter 5.6 for details on using artificial reefs together with transplants.
5.5.1 Mesh mats
Researchers have used this method for transplanting Lessonia nigrescens, Ecklonia radiata, and Phyllospora comosa (42 43, Sydney: Operation Crayweed), but it may be less successful for species that require firm attachment. You first attach plastic mesh mats to the seafloor and then use them as an anchoring point for transplants. Garden mesh (trellis pattern)) is easy to source and relatively inexpensive, and the mesh size can range from 25 to 50 mm. You secure the mesh to the seafloor using pre-installed anchors points (Fig. 5.8). You secure these anchor points by drilling holes into the rock, installing screws and wall plugs or anchor bolts, and screwing/bolting into the plug/anchor.
Once you have created the attachment points in the rock, you should clear the rock of fouling materials, lay the mesh overtop the rock, and fasten the anchors/bolts overtop with a washer to secure the mesh to the rock (Fig. 5.8). There should be a minimum of three attachment points in a triangular formation, but we recommend four or more attachment points (in a square or diamond). You can also use metal bars (three to five cm thick), which effectively act as washers running along the mats to achieve better attachment on the rock. You place these bars between the mesh and the bolt head and should bolt them into the rock every 10 to 20 cm.
Figure 5.8 Mesh mats; illustration by Jon Ferland
After you install the mesh, you secure the transplants using four cable ties per individual. The first cable tie is lightly fastened around the stipe, just above the holdfast. It is important to reduce friction damage to the holdfast by covering this cable tie in rubber tubing. You should loop the three remaining ties through the first and then secure it to the mesh. Work to create a triangular attachment pattern around the holdfast of the kelp.
We recommend plastic or metal due to their longevity as current biodegradable options decompose too quickly. In both instances, there needs to be a plan for removal post restoration.
5.5.2 Green gravel
Also covered in the seeding section, an approach termed “green gravel” (greengravel.org) works by inoculating small stones (i.e., gravel or pebbles) with a spore culture, growing them to juveniles or adults in the lab and then dispersing them into the environment. You can spread green gravel by dropping them off the side of the boat, eliminating the need for scuba divers while increasing scalability and reducing associated costs. If the vector (i.e., gravel) is small enough and there is low wave action, the kelps may overgrow the material and work as a transplantation method, as previously described.
5.5.3 Tiles
Tiles made of clay or other materials can be a convenient method to attach transplants on the seafloor (Italy: Cystoseira). First, you must attach kelps to the tiles, either with rubber bands, adhesives, or naturally cultured on the tile. Tiles can then be secured in the marine environment. You can screw or bolt these tiles into the rock in a similar manner to the mesh mat method described above.
5.5.4 Glues, rubber bands, and holdfasts
Past projects have successfully used the below methods, but they are unlikely to be viable for large-scale restoration projects.
Glue: A small amount of glue, epoxy, or putty may be used to adhere the holdfast to the rock, although it is important to ensure that it can cure underwater (cyanoacrylate, i.e., superglue and marine epoxy work well for this). Ensure that both the holdfast and the rock are clean and dry before using the adhesive. Place firm pressure on the holdfast so that it sets to the rock substrate. If working intertidally, maximize the amount of time the adhesive must set before the tide comes in. This method has achieved some success, but positive results have not been widespread and may carry a high risk of failure.
Rubber bands: Holdfasts can be secured onto existing reef structure using industrial rubber bands 44. Look for rock outcrops that are easy to stretch the band across; conversely this method isn’t feasible if the reef is flat and has no attachment points, or in exposed locations. While low cost, these bands have lasted over two years in sheltered sites but may be less efficacious in wave-exposed sites. This longevity can be beneficial for the longevity of the holdfast, but it is an important point regarding their removal from the ocean following restoration.
Holdfasts: You may use the existing holdfast of another kelp as the attachment points for new transplants. Look to find holdfasts that are still firmly attached to the rock and are near the area you want to restore (i.e., patch edge). You can then tie the transplant onto the existing holdfast, attaching it using industrial rubber bands, cable ties, or thick line.
5.5.5 Line attached to bottom
Seeded lines are often used in aquaculture, but you can also use them in a restoration context. Lines are typically made of nylon, but interest is growing in biodegradable materials as well. Seed lines are either inoculated directly with kelp spores in a culture facility (i.e., direct seeding) or twine/string is first inoculated with spores until reaching a certain size and then wrapped around the larger culture/grow line. You can culture the lines in the lab or in the field. Once the seeded lines are ready for installation, you can anchor them on the sea floor using a similar approach described in the mesh mat section. It is important to use enough attachment points such that the line does not move significantly with the waves. Excessive movement of the lines (side-to-side or up and down) can prevent the holdfast from attaching to the rock.
An alternative approach is to secure wood strips to the seafloor using bolts and nail/staple/screw the line into the wood. This approach results in less movement of the line but requires more time and materials. Select a wood that is rot-resistant and will not decompose quickly in the marine environment (e.g., hardwood).
Table 5.4 Transplantation pros and cons
| Pros | Cons | Reference | |
|---|---|---|---|
| Mesh mats and cable ties | Durable (years) Attach multiple kelps per mat Good for wave exposed areas Applicable for many types of substrates | Potential for plastic pollution Limited to small scales Challenging to install | (Campbell et al., 2014) 45 |
| Gravel | Scalable Lower cost Can seed and culture kelp in lab Diving not required | Kelps may not move from gravel to substrate Vulnerable to wave exposure | (Fredriksen et al., 2020) 46 |
| Glue | Quick Low cost Easy to apply | Sensitive to wave exposure and disturbance | (Westermeier et al., 2014)47 |
| Tiles | Can grow kelps on the tiles No plastic | Fewer kelps per attachment point in the rock Expensive Time intensive | (De La Fuente et al., 2019)48 |
| Existing holdfasts | Low cost Few introduced materials | Relies on existing kelp Not scalable May have competition if attached to a different species | (Hernandez-Carmona et al., 2000)49 |
| Line attached to bottom | Durable Covers large area Line can be seeded and cultured in a lab and/or in marine environment | Kelps may not move from the line to the rock Logistically challenging Expensive Labour intensive | (FIRA, 2020)50 |
| Rubber bands on reef | Low cost Quick | Can be challenging to attach elastic to flat/natural reef substratum Requires divers | (Vásquez and Tala, 1995)51 |
| continued on next page |
5.5.6 Key considerations for transplanting
Transplant density: Ensure sufficient density to help promote recruitment, population growth, and avoid overgrazing. Optimal densities will vary by species and local environmental conditions; aim to mimic densities of naturally occurring populations in the area, although it is worth noting that initial transplanting densities may exceed these values to account for transplant mortality. Density is especially important for intertidal species that are exposed to sunlight and rely on canopy cover to avoid desiccation.
Patch size and shape: At the patch scale (1s-100s of meters), larger patches should help modify the environment to facilitate further growth. Patches with less edge-to-area ratios may also be less susceptible to grazing and disturbance.
Kelp life stage: It is best to collect young kelps (< 1 year) for transplantation as they can have higher growth rates and survivorship than mature plants, and there is evidence that removing large adults can damage wild populations, especially for perennial species.
Patch proximity: At the seascape level (100s-1000m’s of meters), we encourage users to consider creating a network of patches within close proximity in order to better mimic a natural kelp forest. There is evidence that kelp begets kelp, but exact distances and alignments need further testing before we can make more specific recommendations.
Removal of non-degradable materials: Always make sure to remove any materials introduced into the marine environment after the life cycle has completed or the project has expired.
Substrate: Identify and select substrate that is free from competitors such as turf algae, crustose algae, or other marine life (e.g., tunicates, bryozoans, sponges).
Local provenance/origin: Individuals collected from a site may be best adapted at living in those environmental conditions. Aim to match the environmental conditions between donor and transplant sites.
Wave exposure: Selecting a site with reduced wave exposure or days when wave conditions are low can make transplanting when diving considerably easier. Transplants will also be more likely to remain attached at low wave exposure sites.
Timing of outplanting: The ideal time to outplant will be species- and location-specific, but it is preferable to outplant when factors such as water temperatures are low, grazing pressure is low, reproductive output is high, and/ or light intensity encourages growth.
Time out of water: It is important to minimize the time that kelps spend out of the water, while ensuring you keep them cool, wet, and out of direct sunlight (chapter 5.3).
Grazer presence: See chapter 5.2.5
5.6 Artificial reefs
The addition of artificial reef may be required due to lack of kelp habitat, if the habitat was destroyed, or because it is easier to transplant or seed on a reef than on natural substrate. People use artificial reefs to introduce new habitat structure for kelps, other seaweeds, benthic invertebrates, and fishes into the marine environment 52. Smaller reefs may also be used in experiments to test methods and ecological theory 53. Structures are composed of any added hard material: typically concrete but other materials include stones and metals 54.
Artificial reef installation has a long history outside of kelp forest restoration and afforestation. There are numerous legal, environmental, engineering, and logistical considerations required for installing reefs that are outside the scope of this guidebook. The fields of oyster reef restoration and reef fisheries enhancements have made significant progress in addressing these considerations and may be a good point of reference to learn more about the process (chapter 5.6.4). Here we briefly outline the basics of artificial reefs and how they may be used, but further consultation is required before creating a reef.
Reefs are a potentially useful method because they are deployable at an exact location, are raised off the seafloor, and protect kelps from urchin grazing and surface disturbances. They also can be designed to facilitate transplanting and seeding, and they do not require existing natural reef 55. The downsides to using reefs are that they can be very expensive 56, may only cover small areas, may be colonized by species other than kelp 57, and you may face resistance because you are adding materials to the ocean and replacing other habitats (typically sandy bottom). Indeed, installing reefs in areas where kelp has never existed (e.g., sandy bottoms) is afforestation as opposed to restoration. Because reefs can grow new populations that seed nearby populations, we consider them here as a potential tool for restoration.
While there are examples of subtidal reefs being created with a variety of materials (including cars, trains, bombs, and ships in the past), we will only recommend the addition of inert materials designed for the purpose of building a reef (e.g., concrete, stones, and pure metals). We do not advise that you add any unwanted materials to the marine environment. As with any restoration activity, be sure to obtain the proper permits and permissions before commencing work.
Figure 5.9 Concrete reefs; illustration by Jon Ferland
5.6.1 Concrete reefs
Concrete blocks of almost any shape and design may be used to build an artificial reef 5859. You should make sure to elevate the reef off the seafloor to protect kelps from urchin grazing and sedimentation (if needed). You should also select materials that are large enough to withstand wave action and not become dislodged, and be sure to place the reef in a location that minimizes habitat reduction and disruptions to other marine activities. You can add reef blocks to the ocean by using cranes to lower them in off barges, and by using GPS technology you can add them to the ocean with high accuracy 60.
You may modify the structure of the reef such that it is easy to install transplants or seed the area. For instance, you can create slots for substrate containing kelp transplants to be added (Fig. 5.9). These slots or the transplants themselves may be attached on the surface, right before deployment. Attaching the materials above water considerably increases the efficiency of transplanting. For seeding, you can create reefs with attachment points for seed bags to be clipped or tied onto 61. Reefs may also be combined with floating seeded line structures to help seed the surrounding area (Korea: Seaforestation project). Lastly, because increased rugosity helps kelp propagules attach, it can also be beneficial to use a textured surface on the reef structure.
Some projects have experimented with infusing concrete material with nutrients that accelerate kelp growth. Oyamada et al. (2008)62 added these nutrients (iron and nitrogen fertilizers) to the concrete during the manufacturing process, and the nutrients are slowly released as the block weathers underwater. This approach may be useful in areas where nutrients are limiting to growth.
Figure 5.10 Building an artificial reef. Photo supplied by the authors.
5.6.2 Natural substrate
You may also use natural rock material to provide substrate in the ocean. Different compositions such as granite, andesite, basalt, and sandstone—depending on what is natural, available, and cost efficient for the region—are suggested. These stones are added to ocean by pushing them off a barge, either with an excavator or small bulldozer (Fig. 5.10). These materials may be less expensive than using concrete, but they do not allow the specific modifications that concrete structures do. As a result, you should only use rock materials if the goal is to increase the amount of area available for kelp settlement, and you should not consider them an easier way to complete transplants compared to concrete reefs.
5.6.3 Key considerations for artificial reef deployment
Location: It is possible to build a reef with high location specific accuracy; therefore it is worthwhile considering the location of the reef. You may want to place the reef in an area with high kelp settlement, or when installing multiple reefs in an area, you may want to position them so that there is connectivity among the installed populations. For example, when considering kelp recruitment and increasing chances of successful settlement onto the artificial reef, you can use local current models to assess and determine how kelp propagules will travel and float in your restoration location.
Structure size and shape: The materials must be large enough so that they do not move with wave action or during storms. Projects may also construct the structure in order to minimize drag (e.g., with holes) or place it parallel to the prevailing current direction. These steps will stop the reef from eroding as quickly.
Permanency and removal: Because of their size and weight, it is usually cost prohibitive to remove artificial reefs from the ocean. Therefore, you should consider these installations permanent, as reefs will typically last for at least several decades.
Time of deployment and succession: Artificial reefs remain in the water for many years, but it is still worthwhile considering when you add the materials in the marine environment. The installation process requires calm waters for the barges, boats, and cranes to operate, so you should avoid stormy months.
Further, if the reefs are installed without any kelp material, it may be beneficial to install them during the reproduction period of your target species. Following this approach means that the target species are some of the first to settle and grow on the reef. If you miss the reproduction period, other species may colonize the reef first.
Adding kelp to the reef: Transplanting or seeding kelp on the reef at the time of installation may be the best way to ensure the desired kelp species are the first to colonize the available space.
5.6.4 Further reading
63Baine, M. (2001). Artificial reefs: a review of their design, application, management, and performance. Ocean & Coastal Management, 44(3-4), 241-259.
SONGS Artificial Reef, marinemitigation.msi.ucsb.edu/mitigation_projects/artificial_reef/
64 Artificial Reef Subcommittee, Lukens, R. R., & Selberg, C. (2004). Guidelines for marine artificial reef materials. Atlantic and Gulf States Marine Fisheries Commissions, 1-4. 205pp.
65 Fitzsimons, J. A., Branigan, S., Gillies, C. L., Brumbaugh, R. D., Cheng, J., DeAngelis, B. M., ... & Zu Ermgassen, P. S. (2020). Restoring shellfish reefs: Global guidelines for practitioners and scientists. Conservation Science and Practice, 2(6), e198.